Quality Assurance
This article presents the content of Julie's Quality Assurance presentation from the data collection meeting on April 8 2009.
For discussion or questions re. the concepts of QA or Julie's presentation please post your discussions in this article as usual.
What is QA?
An integrated system of activities involving:
- planning
- quality control
- quality assessment
- reporting and
- quality improvement
to ensure that a product or service meets standards of quality with stated levels of confidence.
History of QA
- Started early 19th century in Great Britain during the Industrial Revolution emphasis on product inspection
- Early 20th century by manufacturers and included quality processes
- WWII, US used quality processes in manufacturing of bullets and rifles- sampling technique of inspection
- Japan’s Quality Revolution on automobiles and electronics – focus on improving the organization processes thru people
- 20th century, US introduced Total Quality Management (TQM) – quality on the entire organization
- 21st century, moved beyond manufacturing into service, healthcare, education and government sector
QA in Healthcare
- “All the arrangements and activities that are meant to safeguard, maintain, and promote the quality of care”
- “Set of activities that are carried out to set standards and to monitor and improve performance so that the care provided is as effective and safe as possible”
What is QA to a Medical Database?
- Our product is medical data
- General Goal: To assure the users of these data that they meet standards of quality with stated levels of confidence
- Specific Goal: To determine quantitatively the level of confidence of our data, and to improve that level of quality if needed.
- “Standards” is something set up as a rule for measuring or a model to be followed.
- Quality does not mean “BEST” but may mean “BEST for certain conditions”
- Medically sound, Defensible, Of know quality
- Level of confidence
- Degree of certainty
- Degree of assurance
Principles of QA
- Oriented toward meeting the needs and expectations of the clients
- Focuses on systems and processes
- Uses quantitative data to analyze the problem in the processes
- Encourages a team approach to problem solving and quality improvement
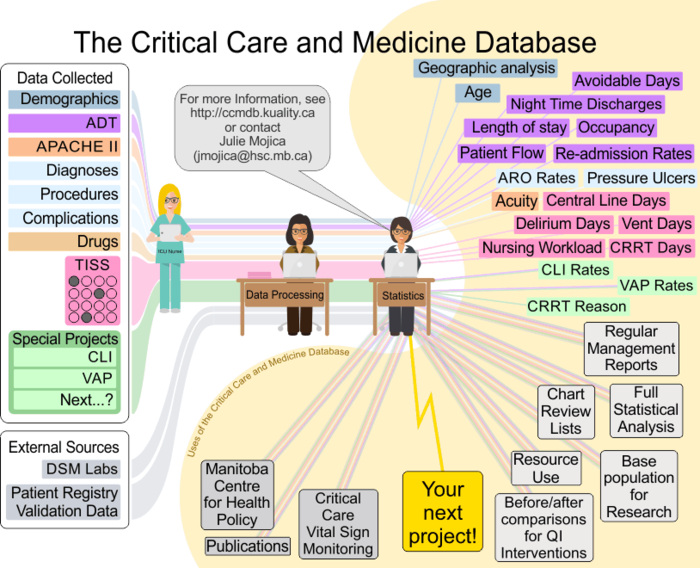
Data Flow at the Critical Care and Medicine Database
Factors Affecting Quality of Data
- Data collection phase
- Sources of data (what, where and how each element is collected)
- Timing of recording
- Hospital protocols/system
- Guidelines (clarity / comprehensive)
- Volume of patients
- Hardwares (PDAs, PCs)
- Memory and capabilities, etc.
- Softwares (Handibase, ACCESS, TMSX & MEDTMS, SAS)
- User friendly/confusing
- Easy/hard to use
- Capabilities in handling large data volume
- Error control features
- Etc.
- Human Factor
- Staff training for new staff
- Staff compliance on the on-going changes in the process
- Staff motivation
- Staff perception on workload
- etc.
Key Elements of QA
- Standard Operating Procedures (SOPs)
- Hardware & Software
- Training
- Statistical considerations
- Monitoring data quality indicators
- Audits
- Documentation
Data Quality Indicators
In assuring the quality of data, the usual concern is to minimize ( or if possible eliminate) errors in the measurement process
Two indicators related in evaluating errors Accuracy and Precision.
Accuracy
Refers to the closeness of the measurement to its true or expected or accepted value.
Precision
Refers to the closeness of the repeated measurements made under the same conditions.
Advantages of QA
- Produces well-documented, reliable and defensible data
- Enhances confidence and credibility of the organization
- Results in efficient use of resources – personnel, equipment and finances
- Improves the staff’s morale and commitment
Conclusion
- QA is not an easy task
- Requires collaboration, cooperation and commitment of staff and management
- Challenge to improve quality of our product